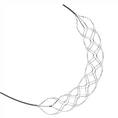
First, stent retriever thrombectomy technology is a representative of mechanical thrombectomy, and revascularization device thrombectomy products are currently the main clinically used thrombectomy devices. With the rapid development of stent retrievers, more and more mechanical clot retrieval device products will enter the market and be used on clinical use. Finer structures and full-length visibility can meet larger thrombus that can penetrate into smaller blood vessels such as the M3 segment of the middle cerebral artery are all future clinical needs. The Dredger Revascularization Device fully developedstent retrieval device independently developed by NeuroSafe Medical Co., Ltd. has an innovative design in terms of full-length visbility, and the excellent visibility under the image can realize precise positioning during the operation. In addition, in terms of revascularization devices specification, NeuroSafe is the only company in China with complete specifications, which meets different clinical thrombectomy needs.

Second, as a new mechanical thrombectomy technique, the aspiration catheter will be more and more widely used in mechanical thrombectomy. In the future, the lumen of the apsiration catheter will become larger to meet the larger thrombectomy requirements of heavy load thrombus. More specifications and models of aspiration catheter can meet the aspiration of smaller size intravascular thrombus. Better catheter flexibility design facilitates the operator to quickly reach the lesion through the tortuous cerebral blood vessels. The Glutton Aspiration Catheter independently developed by NeuroSafe has a 0.072'' ultra-larger lumen with higher aspiration efficiency. In addition, hybraid structure of coil and braiding improves flexibility.